ReActiv8 life
Introducing ReActiv8® – The first & only restorative neurostimulation therapy to treat mechanical chronic low back pain.
The ReActiv8 System is Now Full Body MRI Conditional.
Para os aficionados por jogos de casino que preferem a conveniência de jogar em seus dispositivos móveis, a plataforma app casino dinero real iPhone se destaca como a escolha ideal. Ela oferece uma curadoria dos melhores casinos no Brasil, com aplicativos otimizados para iPhone, garantindo uma experiência de jogo suave e agradável. Estes apps permitem que os usuários desfrutem de seus jogos favoritos com facilidade, seja em casa ou em movimento, combinando alta qualidade gráfica e facilidade de uso. Ideal para quem busca praticidade sem abrir mão da diversão e da chance de ganhos reais.
Para aqueles que buscam soluções inovadoras em saúde, o site https://mainstaymedical.com/ apresenta avanços em neuroestimulação, oferecendo terapias para dor crônica e melhorando a qualidade de vida de pacientes.
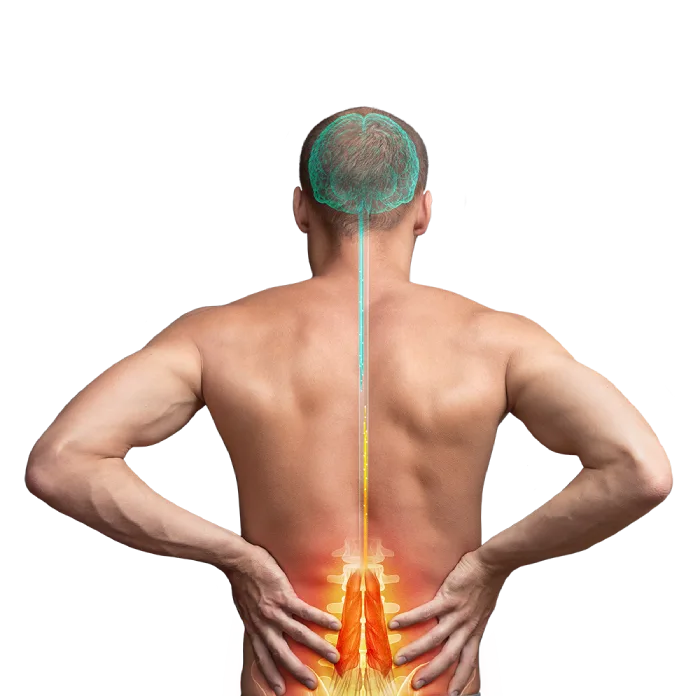
Restorative treatment for your low back pain
For too long, you have suffered from Chronic Low Back Pain (CLBP) and nothing has provided you with lasting or meaningful relief. One reason may be the muscles in your low back that normally prevent painful movements are not working properly. But now you may be able to address this with a revolutionary new device: the ReActiv8 Restorative Neurostimulation system. ReActiv8 is a new restorative therapy that stimulates the nerves of the muscle directly responsible for stabilizing your lumbar spine. The therapy can interrupt your low back’s cycle of pain, degeneration, and loss of function.

Is ReActiv8 right for you? Let’s find out.
Compelling patient outcomes

Treat the cause, not just the symptoms
People experiencing Chronic Low Back Pain have historically had few options: pain medications, physical therapy, and in certain cases, intensive surgery and rehabilitation. These efforts can fail to give many patients meaningful, lasting relief. ReActiv8 is designed to address the underlying cause that can lead to mechanical CLBP and can provide patients a powerful solution that leverages the body’s restorative mechanisms to reduce low back pain.
Restore You
Take the first step to find a doctor that can help determine if ReActiv8 is right for you.


