Introducing Mainstay Medical
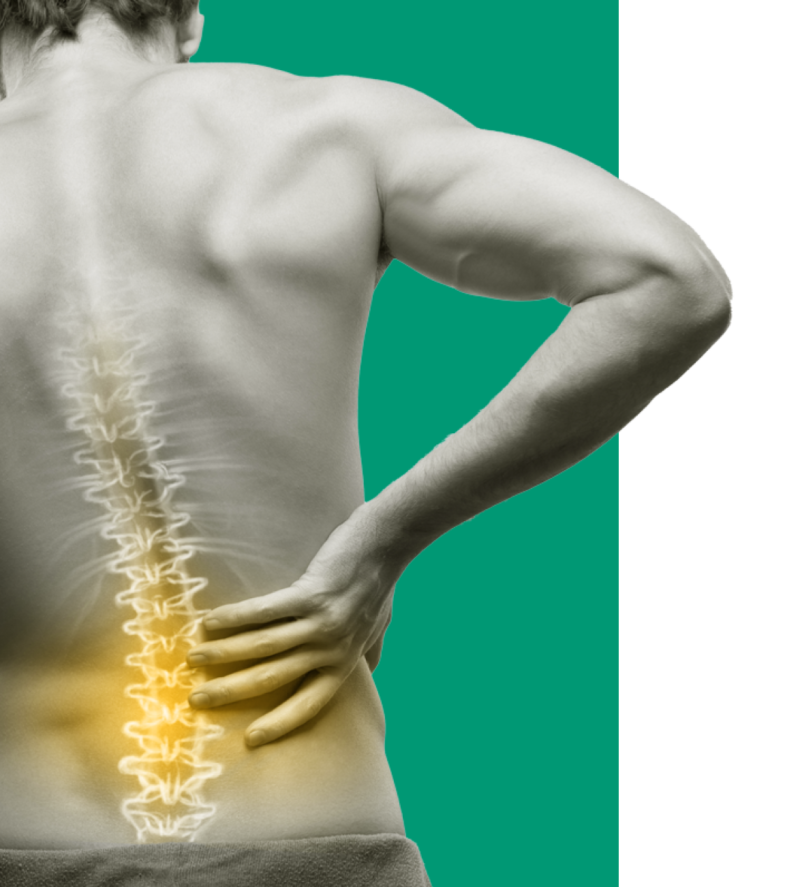
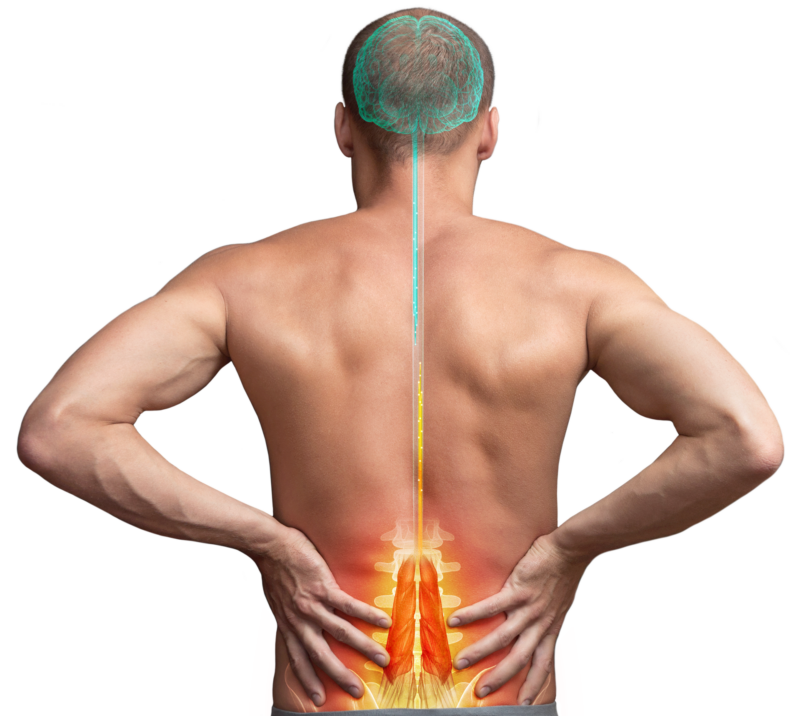
We’ve innovated to deliver a restorative therapy for mechanical Chronic Low Back Pain (CLBP) designed to change patients’ lives.
We are a global medical device company offering therapeutic solutions and new hope for adults suffering from chronic pain. We aim to transform patients’ lives everyday by alleviating pain and improving function.
Mainstay Leadership
Mainstay Medical is led by a team of dedicated scientists, executives, engineers, and clinical experts who leverage their collective experience to develop and commercialize technologies that address unmet clinical needs.
Our story
2019 Mainstay Medical Registration Document Final Summary
2019 Mainstay Medical Final Registration Document
2019 Mainstay Medical Final Securities Note
20 Sep 2019 Mainstay Medical Announces 2019 Half Year Financial Results
20 Sep 2019 Conference call – Friday, 20 September 2019 at 13:00 BST
19 Apr 2019 Mainstay Releases 2018 Annual Report
21 Sep 2018 Mainstay Medical Announces 2018 Half Year Financial Results
21 Sep 2018 Conference call – Friday, 21 September 2018 at 13:00 GMT
29 Jun 2021 Mainstay Medical Announces Limited Commercial Launch of ReActiv8 in the U.S.
08 Mar 2021 Mainstay Medical Announces Commercial Launch of ReActiv8 in Australia
16 Feb 2021 Mainstay Medical Announces US$108 Million Equity Financing Transaction
25 Jan 2021 Mainstay Medical Announces Key Strategic Hires to Drive Global Commercial Expansion
28 Jul 2020 Mainstay Medical to Present at the LifeSci Partners Summer Symposium
09 Jun 2020 Mainstay Medical Announces Reimbursement Approval for ReActiv8 in Australia
05 Jun 2020 Mainstay Medical announces Scheme becoming effective
04 Jun 2020 Receipt of High Court approval in connection with proposed Reorganization
02 Jun 2020 Mainstay Medical announces expected timetable for completion of Reorganization
25 May 2020 Mainstay Medical announces Date for Court Hearing
08 May 2020 Mainstay Medical announces Results of Scheme Meeting and EGM
25 Oct 2019 Mainstay Medical Announce Publication of Prospectus and Admission
20 Sep 2019 Mainstay Medical Announces 2019 Half Year Financial Results
12 Sep 2019 Mainstay Medical Announces Date for 2019 Half Year Results
30 Jul 2019 Mainstay Medical Announces Completion of Financing Transactions
19 Nov 2018 Mainstay Medical Announces Headline Results from ReActiv8-B Clinical Study
19 Nov 2018 ReActiv8-B Results Presentation for Conference Call and Webcast
24 Sep 2018 Mainstay Medical Announces Board Change
21 Sep 2018 Mainstay Medical Announces 2018 Half Year Financial Results
04 Sep 2018 Mainstay Medical Announces Date for 2018 Half Year Results
20 Aug 2018 Mainstay Medical Announces the Appointment of Matthew Onaitis as Chief Financial Officer
09 Jul 2018 Mainstay Medical announces the completion of all implants in the ReActiv8-B Study
20 Jun 2018 Mainstay Medical Appoints Wolfgang Frisch as VP and Managing Director for Germany
22 May 2018 Mainstay Medical Announces CFO Transition
04 May 2018 Mainstay Medical Announces Publication of Prospectus and Admission
05 Apr 2018 Update on Regulatory Application for ReActiv8 in Australia
15 Feb 2018 Mainstay Medical Announces €30 million Financing
23 Jan 2018 Mainstay confirms its continued eligibility to the PEA-PME
11 Dec 2017 Mainstay Medical Announces Positive Outcome of Interim Analysis
09 Oct 2017 Mainstay Medical Announces Jason Hannon Joins Board of Directors
05 Sep 2017 Mainstay Medical Announces CEO Leadership Transition
05 Sep 2017 Mainstay Medical Announces 2017 Half Year Financial Results
04 Sep 2017 Mainstay Medical Announces Date for 2017 Half Year Results
03 Aug 2017 Mainstay Medical’s ReActiv8-B Clinical Trial Passes Mid-point
15 Jun 2017 Mainstay Medical to Present at the JMP Securities 2017 Life Sciences Conference
31 May 2017 Mainstay Medical to Present at the Jefferies 2017 Global Healthcare Conference
16 May 2017 Mainstay Medical to Present at the UBS Global Healthcare Conference
28 Apr 2017 Mainstay Medical to Present at the Deutsche Bank 42nd Annual Health Care Conference
19 Apr 2017 Mainstay Medical to Present at the MedTech Strategist Innovation Summit: Dublin 2017
31 Mar 2017 Mainstay Medical to Present at the 24th MedTech Investing Europe Conference
23 Mar 2017 Mainstay Medical Publishes 2016 Full Year Results and Business Update
16 Mar 2017 Mainstay Medical Announces Date for 2016 Full Year Results
07 Mar 2017 Mainstay Medical to Present at the Canaccord Genuity 2017 Musculoskeletal Conference
24 Jan 2017 Mainstay confirms its continued eligibility to the PEA-PME
12 Jan 2017 Mainstay Medical Applies for Approval to Market ReActiv8® in Australia
06 Jan 2017 Mainstay Medical to Present at Medtech Showcase 2017
26 Oct 2016 Mainstay Medical Announces Additional U.S. Patent Issued
22 Sep 2016 Mainstay Medical Announces 2016 Half Year Financial Results
20 Sep 2016 ReActiv8-A Clinical Trial – Sustained Performance at One Year
12 Sep 2016 Mainstay Medical Announces Date for 2016 Half Year Results
11 Aug 2016 Mainstay Medical Announces Publication of Prospectus and Admission
11 Aug 2016 Mainstay Medical to Present at Wedbush PacGrow 2016 Healthcare Conference
20 Jun 2016 Mainstay Medical to present at the 2016 JMP Securities Life Sciences Conference
17 Jun 2016 Mainstay Medical Announces €30 million Fundraising
25 May 2016 Mainstay Medical Achieves CE Marking for ReActiv8®
28 Apr 2016 Mainstay Medical Publishes its 2015 Annual Report
27 Apr 2016 Mainstay confirms its continued eligibility to the PEA-PME
07 Apr 2016 Mainstay Medical to present at Innovation in Medtech Dublin 2016 Conference
08 Feb 2016 Mainstay Medical Full Year 2015 Preliminary Results and Business Update
03 Feb 2016 Mainstay Medical Announces Date for 2015 Preliminary Results
The shareholder conference call of the Extraordinary General Meeting of Mainstay Medical Holdings PLC is scheduled to commence on the 23rd of February 2024. Documents to Review: Reinstated Constitution of Mainstay Medical Holdings PLC
Time of Conference:
4PM GMT (Ireland)
11AM ET/10AM CT (USA)
01 Oct 2019 Holding(s) in Company
01 Oct 2019 Holding(s) in Company
27 Aug 2019 Notification of Transaction of PDMR
13 Aug 2019 Total Voting Rights
06 Aug 2019 Holding(s) in Company
06 Aug 2019 Holding(s) in Company
06 Aug 2019 Holding(s) in Company
31 Jul 2019 Notification of Transaction of PDMR
31 Jul 2019 Notification of Transaction of PDMR
01 Mar 2019 Total Voting Rights
01 Feb 2019 Notification of Transaction of PDMR
03 Dec 2018 Block Listing Return
31 Oct 2018 Notice of change of registered office
29 Aug 2018 Total Voting Rights
01 Jun 2018 Block Listing Return
28 Mar 2018 Notification of Transaction of PDMR
01 Mar 2018 Total Voting Rights
19 Feb 2018 Holding(s) in Company
19 Feb 2018 Holding(s) in Company
19 Feb 2018 Holding(s) in Company
19 Feb 2018 Holding(s) in Company
19 Feb 2018 Holding(s) in Company
19 Feb 2018 Notification of Transaction of PDMR
19 Feb 2018 Notification of Transaction of PDMR
19 Feb 2018 Notification of Transaction of PDMR
19 Feb 2018 Notification of Transaction of PDMR
19 Feb 2018 Notification of Transaction of PDMR
16 Dec 2016 Notification of Transaction of PDMR
16 Dec 2016 Notification of Transaction of PDMR
16 Dec 2016 Notification of Transaction of PDMR
16 Dec 2016 Notification of Transaction of PDMR
01 Dec 2016 Total Voting Rights
01 Dec 2016 Block Listing Return
01 Nov 2016 Total Voting Rights
03 Oct 2016 Total Voting Rights
29 Jul 2016 Total Voting Rights
06 Jul 2016 Holding(s) in Company
01 Jul 2016 Total Voting Rights
21 Jun 2016 Holding(s) in Company
21 Jun 2016 Holding(s) in Company
21 Jun 2016 Director/PDMR Shareholding
21 Jun 2016 Director/PDMR Shareholding
21 Jun 2016 Director/PDMR Shareholding
21 Jun 2016 Holding(s) in Company
21 Jun 2016 Holding(s) in Company
21 Jun 2016 Holding(s) in Company
21 Jun 2016 Holding(s) in Company
21 Jun 2016 Holding(s) in Company
21 Jun 2016 Holding(s) in Company
21 Jun 2016 Holding(s) in Company
01 Jun 2016 Blocklisting Return
01 Apr 2016 Total Voting Rights
01 Mar 2016 Total Voting Rights
02 Feb 2016 Blocklisting Announcement
Corporate Governance
The Board recognizes the importance of good governance in supporting growth in long term shareholder value and is accordingly committed to maintaining the highest standards of corporate governance commensurate with the size and stage of the development of the Group.
While there is no specific corporate governance regime mandated in Ireland for companies listed on ESM nor is there any specific corporate governance regime mandated in France for companies who are listed on Euronext but not incorporated in France, the Company applies recognized corporate governance principles to the extent they are appropriate for a company of its size, stage of development and resources.
The Board will also take account of other institutional shareholder governance guidelines on disclosure and shareholder authorizations to the extent they are appropriate for a company of its size, stage of development and resources.
The Board of Directors
The Board is responsible for the supervision and control of the Company and is accountable to the Company. The Board has reserved decision-making on a variety of matters, including determining strategy for the Group, reviewing and monitoring executive management performance and monitoring risks and controls.
The Board comprises nine Directors, including one Executive Director, seven Non-Executive Directors and the Non-Executive Chairman. The roles of Chairman and Chief Executive Officer are not exercised by the same individual.
The Board meets regularly (no less than four times per year) to consider strategy, performance and the framework of internal controls. The Directors have also established an Audit, Risk and Compliance Committee, a Remuneration Committee, and a Nominations Committee with formally delegated rules and responsibilities. Each of the Committees currently comprises Non-Executive Directors only.
The Board comprises a mix of the necessary skills, knowledge and experience required to provide leadership, control and oversight of the management of the Company and to contribute to the development and implementation of the Company’s strategy. In particular, the Board combines a group of Directors with diverse backgrounds within the medical device and related sectors, in both public and private companies.
All the Directors bring independent judgment to bear on issues affecting the Group and all have full and timely access to information necessary to enable them to discharge their duties. The Articles require each Director retire at the annual general meeting held in the third calendar year following the year in which he was appointed or last re-appointed but unless he falls within the paragraph immediately below he shall be eligible for re-appointment.
A Director shall also retire at any annual general meeting if he has agreed to do so (whether in accordance with the terms of his appointment or otherwise) and, unless the Directors have agreed otherwise, he shall not be eligible for re-appointment.
This section serves as notice under 35 U.S.C. §287(a) that the following products are protected by one or more U.S. patents. Each product also may be covered by one or more foreign patents, and additional patent application(s) may be pending.
The following list of products and U.S. patents may not be all inclusive, and other products not listed here may be protected by one or more patents.
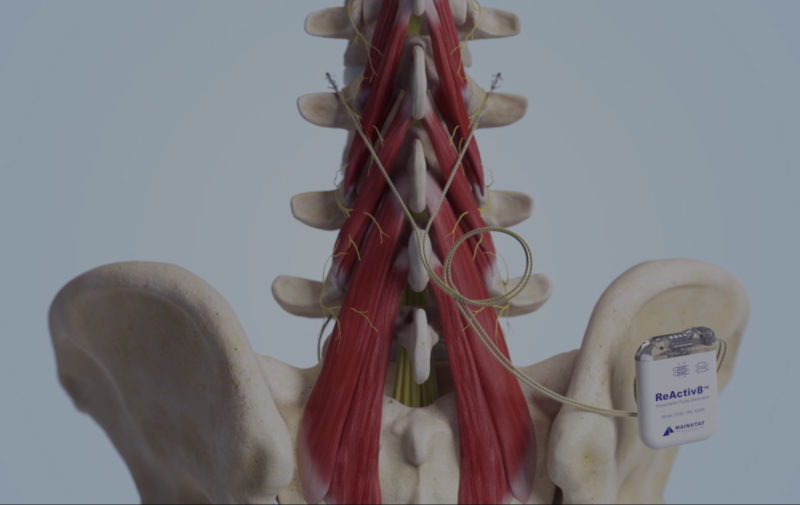
ReActiv8 is covered by one or more of the following U.S. patents: 8,428,728; 8,606,358; 9,072,897; 9,474,906; 9,861,811; 9,999,763; 10,016,603; 10,195,419; 10,327,810; 10,449,355; 10,828,490; 10,925,637; 11,103,706; 11,331,488; 11,471,670; 11,679,261; 11,679,262; 11,684,774, 11,786,725 as well as their international equivalents. Other patents pending.

An innovative, patient-first culture
We’re a growing company of enthusiastic team players who enjoy collaboration, value relationships, and want to challenge themselves every day. Join us in helping others rediscover living. Learn more about our innovative, patient-first culture click here.